Epigenetic in heart
Ageing is often associated with an impairment of heart function that can lead to heart failure, the primary cause of disability and death in the elderly. Although vascular abnormalities associated with aging (e.g., hypertension and atherosclerosis) contribute to age-related cardiac malfunctioning, myocardium-remodelling is also involved. The molecular mechanisms underlying this process are not completely understood. However, gene expression analysis has revealed that cardiac aging is accompanied by changes of gene expression. These findings support the hypothesis that cardiac remodelling occurring in aging is caused by a reprogramming of the expression, which in many respects is similar to that observed in stressed heart.
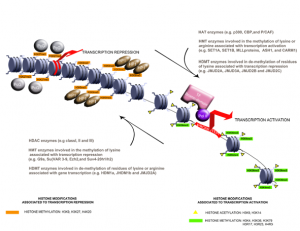
An exciting finding of the past 20 years is that transcription regulation in eukaryotic cells depends not solely on elements of DNA that control gene expression (e.g. promoter and enhancer sequences) but also on the state of the chromatin in which a gene is located. In this scenario, the main player is epigenetics ― a complex network of mechanisms that controls gene expression in a potentially heritable way but without altering the primary nucleotide sequence. These mechanisms regulate gene expression by modulating chromatin structure and DNA-based biological processes, such as the binding of transcription factors to promoters and transcription elongation (Fig. 1). Epigenetic mechanisms can be grouped into four main categories: DNA methylation, histone modifications, nucleosome positioning, and regulation by small RNAs.
Fig. 1 Example of histone modification signatures involved in the regulation of gene transcription [Source: Papait R et al., Chapter 24 of book Muscle, to be published by Elsevier]
Recent studies showed the importance of histone modifications and small RNAs in defining the gene expression changes causing heart failure.
Despite this, the involvement of epigenetics in cardiac aging has been largely discussed but poorly studied.
We are a young group that has the ambitious project of elucidating the role of histone modifications in the regulation of gene expression programming during cardiac aging. We aim to map the epigenome and understand the function of epigenetic enzymes in these processes, through the combination of high-throughput approaches used for studying the epigenome and traditional methodologies employed for studying cardiovascular disease.
Methods:
- embedding, sectioning, staining and recognition of histological preparation by optical and electronic microscopy (TEM);
- immunogold and immunofluorescence techniques;
- extraction, quantification, and separation of protein;
- extraction and quantification of DNA;
- ChIP-Seq and RNA-Seq;
- metabolites extraction and colorimetric/fluorometric metabolic assays for their quantification.
https://www.humanitas-research.org/groups/gianluigi-condorelli-group/